Post-Finasteride Syndrome: A dangerous situation creating a hidden public health crisis
Post-Finasteride Syndrome is a serious clinical problem with no effective treatment that is causing severe and permanent health problems in a subset of men.
While this is the sober reality facing patients experiencing the disease, a perfect storm of regulatory, commercial and clinical factors is creating a dangerous situation where:
The safety information available to potential consumers of the drug is woefully inadequate
Those unlucky enough to develop Post-Finasteride Syndrome are met with a clinical environment that is in the best cases unhelpful, and in the worst cases negligent or hostile
Visibility of the disease remains remarkably poor, delaying scientific discovery and effective treatments for suffering patients
Regulatory action is almost completely absent, and men continue to go mostly unwarned about persistent harm which can occur after stopping finasteride
Each of these interdependent factors has serious consequences, making Post-Finasteride Syndrome a hidden public health crisis which must be urgently addressed.
Information is biased and erroneous, and warnings are misleading
Biased and erroneous information about the safety of finasteride and a misleading warning label create an environment where consumers cannot provide informed consent to the drug’s risks.
This situation is driven by two key factors:
Financial conflicts of interest for those providing safety information about the drug
An absence of appropriate regulatory action
According to a 2019 study, 68.9% of Americans used the internet first in their most recent search for healthcare information. This experience is also largely representative of consumers of finasteride, who use online sources such as hair loss forums, YouTube and Reddit to seek information about the drug.
“After my hair loss started to bother me in my late 20’s, I would check online, periodically, for information about finasteride and whether I should take it or not.”
PFS patient Mike, PFSN Podcast Series
Much of this content, however, is created by those with commercial interests. According to a 2020 study published in the Aesthetic Plastic Surgery Journal, online content about hair loss treatments is often biased and misleading. The study evaluated YouTube videos using the DISCERN criterion, a tool that assesses the quality of consumer health information. A score of 1 indicates a low overall quality whereas 5 indicates a high-quality source of information for patients.
Assessing 90 videos about hair loss treatment the study found YouTube influencers represented 37.8% of authors followed by companies and advertisers with 15.6%. The overall quality score was 2.66 and the bias score 2.98, below the mean for both criteria.
A prime example of biased and misleading content is the top search result for Post-Finasteride Syndrome on YouTube. The authors, two Australian hair loss doctors, omit key medical literature about PFS and make erroneous statements about when persistent side effects were first reported. The assumptions and statements provided by the presenters are based almost entirely upon their anecdotal experience and are not reflected in either the 20 year record of patient self-reports or the majority of medical literature.
Of even greater concern is that they provide no indication of what a patient should do if they experience side effects that persist. The video description contains a link to their hair loss clinic.
Other examples of erroneous, biased, or misleading information include:
The finasteride page for online health company, Roman, misleads consumers by saying that side effects were only experienced in men taking the 5mg dose, rather than 1mg. This is completely false.
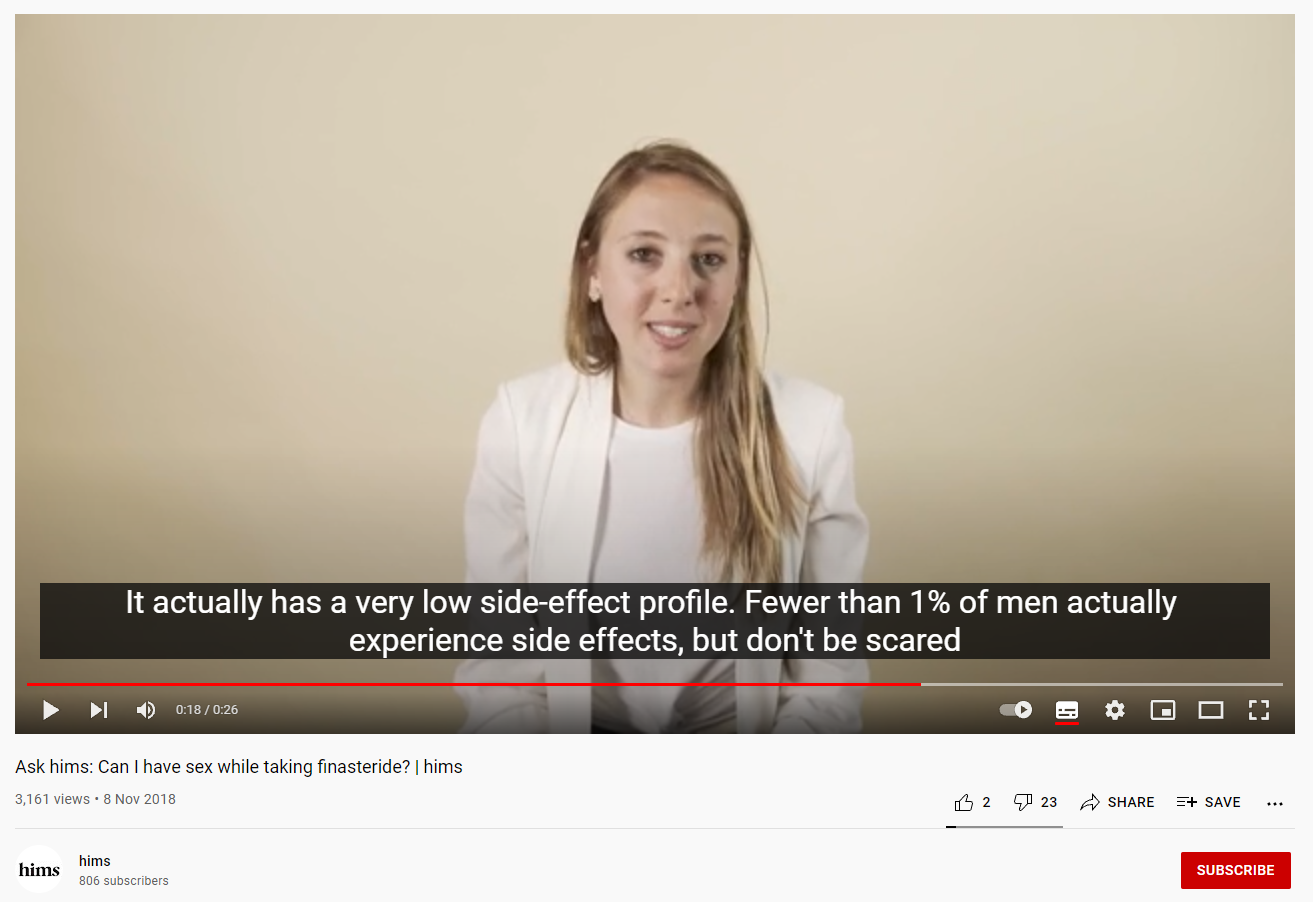
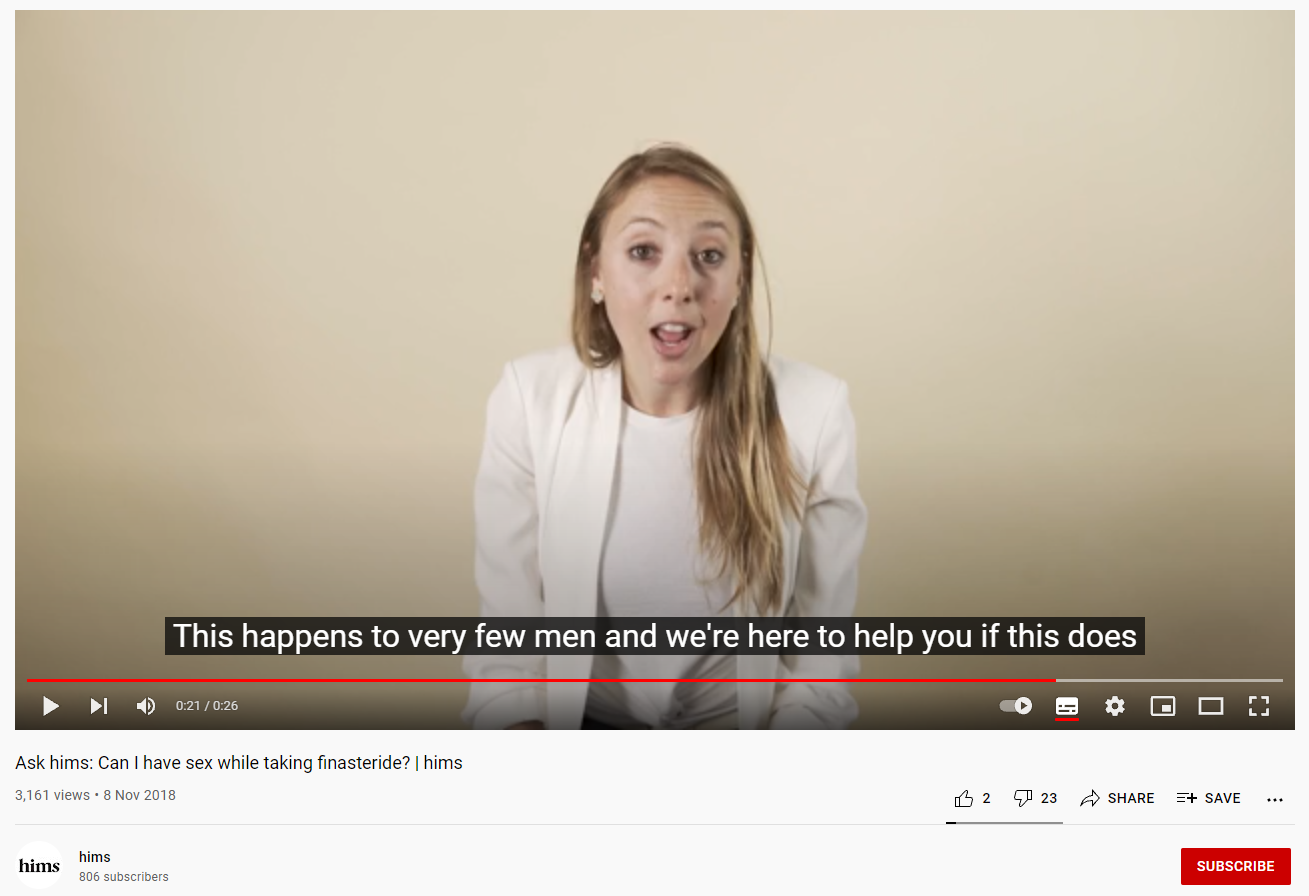
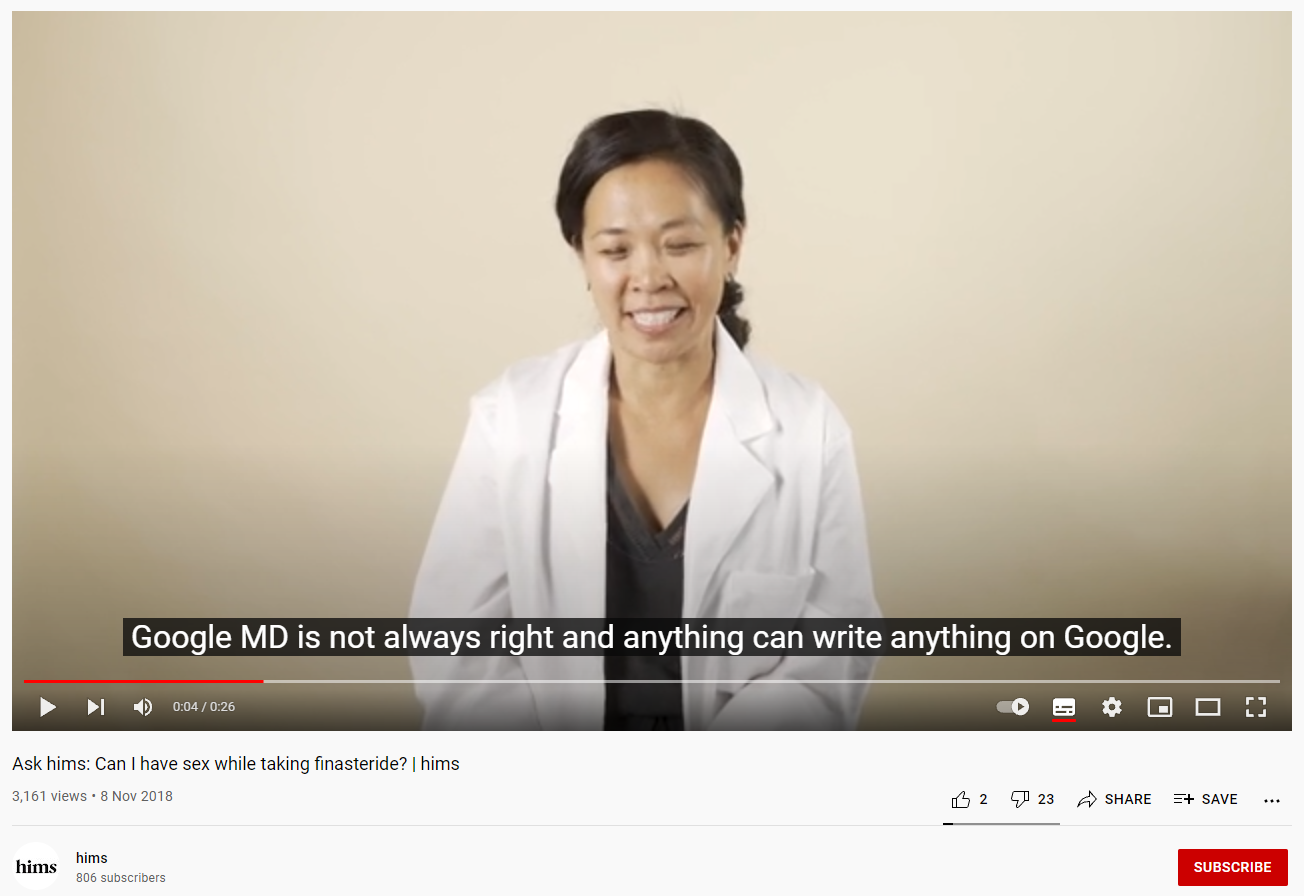
A 2018 YouTube video by online health company, Hims, shows a young woman in a lab coat visibly laughing while saying “anything (sic) can write anything on google”. Another woman, also wearing a lab coat, assures consumers that “fewer than 1% of men actually experience side effects”, which is completely inaccurate. The warning label for finasteride states sexual dysfunction occurred in 3.8% of men during clinical trials.
A blog by online hair-loss company Keeps about finasteride’s side effects contains numerous issues. Its opening sentence states there a lot of “rumours” about finasteride and its side effects. Dozens of published scientific studies and the manufacturer’s own warnings cannot be described as “rumours”. It features hair-loss doctor Dr Jerry Shapiro completely dismissing the possibility of permanent side effects. Dr Shapiro is both employed by Keeps as an advisor, and has previously attempted to misrepresent Merck’s clinical trials data about persistent sexual dysfunction, raising questions about his appropriateness to make such claims. The article also states that hair loss experts agree there is no evidence finasteride can cause permanent effects, despite case-controlled scientific studies in both humans and animals suggesting otherwise.
These are only a few examples where those who stand to financially gain from the sale of finasteride provide misleading, biased or erroneous information about its safety risks. As we wrote in our 2020 literature review, those promoting finasteride as a safe product for young men without warning of possible permanent side effects can easily be likened to tobacco executives of the 1980s.
Some consumers may learn about the drug elsewhere and rely on information from a clinician, such as a general practitioner, dermatologist, or hair loss specialist. Dermatologists and hair loss clinics are aware that finasteride, or other anti-androgenic substances like dutasteride, are the only drugs that can effectively stop hair loss. Such reliance creates an inherent conflict of interest regarding how safety information is presented.
This doesn’t mean that all clinicians are maliciously withholding information from their patients.
Most clinicians will provide their patient with safety information from two key sources: the drug’s clinical trials, and the warning label. As we covered recently, this warning label has been proven to be deceptive and misleading, a fact many clinicians are unaware of.
Our video covers a 2019 Reuters investigation, which showed the manufacturer of Propecia, Merck, deliberately withheld information that demonstrated the existence of persistent side effects in at least one man, and potentially as many as seven, during 5-year clinical trials in the 1990’s. Internal Merck memos, emails and depositions from a U.S litigation showed high-ranking executives accepted this evidence, and subsequently made subtle and misleading changes to the warning label. The most deceptive: a 2002 update from “side effects resolved in all men who discontinued Propecia” to “side effects resolved in men who discontinued Propecia”.
Despite this, regulatory bodies have failed to act and the warning label remains the same. It is completely misleading, as it implies side effects will resolve for all men while failing to provide clinicians or consumers important context on why the word “all” was removed. Due to poor visibility of the investigation, many clinicians are still unaware of this information.
This trial data and the warning label are often cited in online sources as proof finasteride is well-tolerated. As regulatory bodies have failed to act, this practice is allowed to continue. Further, regulatory bodies have also largely failed to act on more than 25,000 global adverse event reports and dozens of published scientific studies.
Faced with such an overwhelming body of misleading information about the drug’s safety risks, patients cannot possibly provide informed consent, which was the case for all of our guests on our recent podcast series.
“No, I didn’t have enough information at all. Not even close.”
PFS patient Ardalan, PFSN Podcast Series
The emergence of online prescriptions and potential dangers
Since the expiration of Merck’s patent on finasteride, a new type of business has taken advantage of the ability to sell cheaper generics to patients online. Companies such as Keeps, Hims and Roman are now able to prescribe finasteride quickly and cheaply.
The high stakes involved in the hair loss industry and the sale of finasteride.
One problem with this new competitive environment is that these companies are not “Mum & Dad” dermatology practices. They are not small businesses, and the nature of their funding models, where they receive the majority of pre-IPO capital from venture capital firms expecting a high ROI, creates conflicts of interest regarding the presentation of drug safety information. Once these companies are publicly traded, shareholders expect consistent growth, or they risk a diminishing share price, which creates further pressure to sell more product.
These companies also position themselves as technology companies and not healthcare providers. They provide a platform for consumers to access prescription medications, and claim they do not provide healthcare advice themselves. As previously demonstrated, however, these companies will downplay drug safety risks in blog articles and other content that serves a commercial purpose.
In the absence of tighter regulation, consumers are left to receive advice about a drug’s safety that amounts to mischaracterisations of a highly complex disease by marketing professionals, and anecdotal evidence from hair loss doctors with a vested interest in the sale of the drug.
“This (buying finasteride online) is a huge problem, as the goal of the company is to simply sell as much of the product as possible. The priority of these businesses is profit over patient safety.
I strongly advise patients to avoid such websites and clinics that promote a particular medication with claims that are not based upon scientific study.”
Endocrinologist, Dr Michael Irwig, PFSN Podcast Series
Another problem is their use of aggressive online advertising tactics. While drug manufacturer Merck owned the patent on Propecia, the drug was marketed much differently. Consumers may have learned about it through a friend or simply consulted with their GP or dermatologist.
Now, telehealth companies aggressively market hair loss medications online through TV commercials, social media, paid search ads and influencers. They sponsor popular YouTube channels with primarily young male audiences. The framing and messaging of these ads is remarkably similar to how women’s cosmetic products are offered, creating and subsequently targeting a body-image problem for men.
Telehealth companies are aggressively marketing finasteride to men through new channels with huge audiences.
As finasteride features prominently in these companies’ product suites, they cannot reasonably be expected to be forthcoming with consumers about its potential life-altering consequences.
It’s no coincidence that this new environment has seen a surge in annual finasteride prescriptions. According to health data company IQVIA, the total number of patients accessing finasteride in the United States alone increased to 2.4 million in 2020, more than double the number in 2015. With new patient delivery models that prioritise convenience and speed contributing to widespread proliferation of the drug, and an absence of regulatory activity, more men will continue to develop Post-Finasteride Syndrome.
An unsupportive clinical environment
If a consumer is unlucky enough to develop Post-Finasteride Syndrome, they are faced with a clinical environment that is largely unhelpful. Due to a lack of clear biomarkers, patients are often met with outward scepticism of the disease’s existence by clinicians and sometimes even harmfully misdiagnosed as delusional.
This experience is supported by a 2014 study from Dr Christine Ganzer, who noted an ‘alarming dissatisfaction’ in the clinical experiences of PFS patients surveyed. Literature reviews from Professor Abdulgamed Traish have noted an absence of awareness and poor understanding of the condition among clinicians, despite an emerging body of medical literature suggesting an epigenetic susceptibility in a subset of patients. Our own patient survey found the average score for what patients describe as their most positive clinical experience was between dissatisfaction and serious dissatisfaction.
According to Dr Irwig, one of the contributing factors to poor awareness and understanding is that many physicians received their training before the emerging body of scientific evidence was published, such as Baylor College of Medicine’s 2021 findings of differential gene expression in PFS patients, their 2019 study which demonstrated penile vascular abnormalities, and other studies you can find on our science page. Some of the studies, such as one from The University of Milano, have been in circulation since 2015.
Another factor contributing to inadequate patient care is a 2019 report from Swiss dermatologist Dr Ralph Trueb, which mischaracterised Post-Finasteride Syndrome as a delusional disorder. There are several notable problems with Dr Trueb’s study, including:
It was based on a single patient report, and the patient had no documented depressive history.
Dr Trueb suggested an airing of a Swiss documentary had caused “mass hysteria” that led to the development of a psychosomatic condition. However, by Dr Trueb’s own definition of mass hysteria as “many people believing obviously false and potentially distressing things based purely on hearsay”, this cannot be the case due to basic science and animal research, as well as the outcomes of several case-controlled studies of PFS patients, which are not addressed.
Dr Trueb’s dermatology clinic specialises in hair loss and has prescribed finasteride for nearly two decades. Despite this, he has declared no conflict of interest.
Due to a lack of clinical awareness, when patients present reporting persistent side effects, many practitioners seek information about PFS online. And despite a large body of supporting medical literature, they will find Dr Trueb’s report as the top Google search result for Post-Finasteride Syndrome. Sadly, the prominence of this article also contributes to dismissal or denial of symptoms from the loved ones of patients, increasing the trauma experienced when seeking clinical support.
“When I told my family about PFS, the first thing they did was Google it, and found this article calling us delusional. It put doubt in their minds.”
PFS patient Aksh, PFSN Podcast Series
The dismissal of Post-Finasteride Syndrome by physicians results in a compounding of clinical failures, as adverse reactions often go unreported. According to FAERS, the Food and Drug Administration’s adverse events reporting system, there have been 18,813 adverse event reports related to finasteride. Alarmingly, only 33% of these reports were submitted by a healthcare professional. Comparatively, 67% of adverse event reports for Aspirin were submitted by a healthcare professional.
This is a significant problem, as regulatory agencies such as the FDA and European Medicines Agency rely on frontline reporting. A lack of accurate reporting leads to an absence of regulatory action, improved warning labels, and importantly, scientific investigation, contributing to a cycle of neglect that leaves potential consumers at risk.
PFS patient Damon, who we spoke to on our podcast series, experienced extreme physical changes at age 23 such as muscle wastage, thinning and loosening of skin, and muscle atrophy, problems he had never experienced as an avid bodybuilder. His experience was completely dismissed by a treating physician, and misdiagnosed as depression.
“I remember showing him about four different studies about finasteride’s effects, and he didn’t even look at them. And this was a board-certified neurologist. He said “good job on the studies, but that’s probably more long-term use”. After that he just completely dismissed it and gave me an anti-depressant.”
An obscure corner of the internet
Faced with neglect and stigma, internet resources like propeciahelp are left to shoulder an unsustainable clinical burden.
When saddled with such significant disability, individuals find hope in the form of often-dangerous self-experimentation. Vulnerable patients are open to exploitation by individuals or businesses offering simplistic explanations and suggesting treatments. This is particularly problematic as patients with a novel fragility to further disruption of the androgen pathway are susceptible to permanent worsening, with evidence that such experimentation directly preceded completed suicide.
As patients retreat to the anonymity of online forums, visibility of their records remains alarmingly low. Despite nearly 7000 registrations to the propeciahelp forum and two decades of self-reports, less than two dozen patients have spoken publicly. This culture of anonymity becomes an interdependent factor with clinical neglect and stigmatisation, further contributing to poor awareness, education and dismissal of the disease.
Consistent and appropriate scientific investigation sorely lacking
It should also be noted that without appropriate clinical acknowledgement, reporting, regulatory action and patient visibility, progression of scientific understanding has been slow. This becomes another interdependent factor which further fuels this hidden crisis.
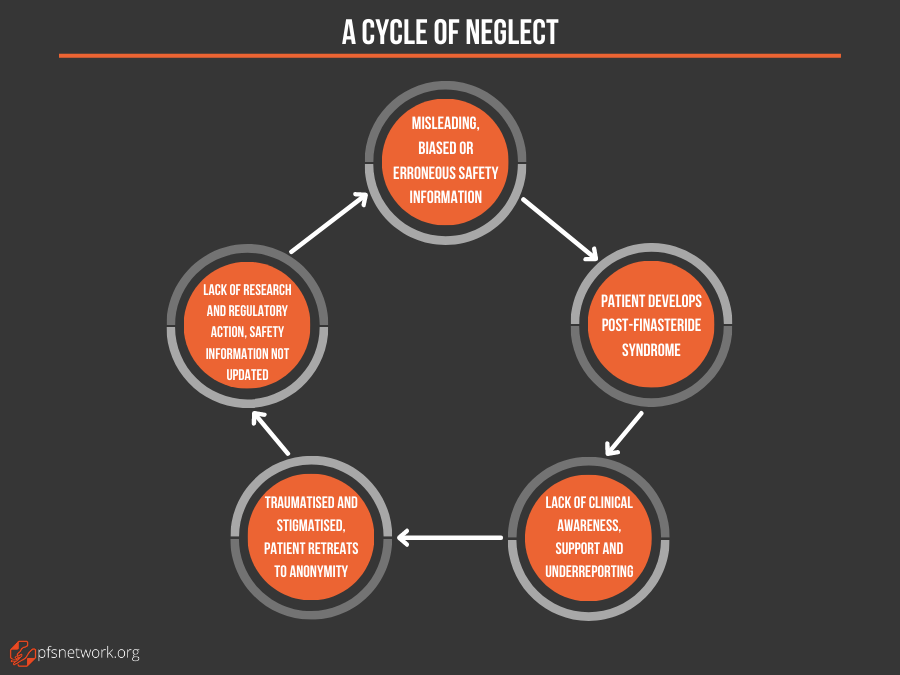
Men predisposed to finasteride’s permanent harm are dangerously at risk due to a cycle of neglect.
Significant scientific interest occurs when there appears to be a serious problem, and when there appears to be none, that interest is scarce. Without further scientific understanding, there remains room for a serious health problem to be obfuscated, and the harmful practices mentioned in this article to continue.
A hidden public health crisis and the unacceptable human toll
Importantly, these factors are interdependent, creating a cycle of neglect that will continue unless addressed.
The end result is a hidden public health crisis with an unacceptable human toll.
The majority of men developing Post-Finasteride Syndrome are young and particularly susceptible to these systemic failures. Left with significant physiological damage, many are unable to continue working or studying and rely on family and friends for support.
In the worst cases patients are taking their own lives, leaving behind broken families. Documented suicides, such as Daniel Stewart, Conall Gould, John Pfaff and Randy Santmann, were once happy and healthy men. Alarmingly, there have been 614 FAERS reports of suicidality from those taking finasteride, including 110 deaths, and 2830 reports of suicidality globally. However, it is impossible to accurately define a number of completed suicides, as those experiencing the most severe problems can disappear suddenly and without trace after expressing suicidality with their cases improperly recorded.
The number of patients accessing finasteride in a single developed nation has more than doubled in five years and will continue to grow. And unless these factors are urgently addressed, a hidden public health crisis will continue to insidiously grow as well.